Rhizomatic Growth of Woody Bamboo
Updated: Jun 12, 2024

We're excited to share a recent scientific paper by our own agronomist, Hormilson Cruz, originally published in the "International Journal of Agriculture, Environment and Bioresearch"! This article dives into the fascinating world of sympodial bamboos, a unique species known for their non-invasive growth patterns.
This research is particularly relevant to our ongoing project at bamboo bioproducts, involving the sustainable cultivation of Bambusa vulgaris var vulgaris, a sympodial bamboo often mistakenly considered invasive. This article provides valuable insights to dispel this misconception and showcases the benefits of responsible bamboo management.
RHIZOMATIC GROWTH OF WOODY BAMBOO
ABSTRACT
Bamboos, ancient plants that have thrived sustainably for centuries, contribute significantly to the regions where they naturally grow, particularly in countries across the eastern hemisphere. Their presence worldwide is primarily associated with natural forests, where they have been utilized in various economic activities without significant environmental controversies. These forests have evolved naturally in an endemic or native manner. However, the recent surge in commercial bamboo farms has posed a dilemma for government entities responsible for approving or disapproving their establishment, sparking a contentious debate over whether bamboo is invasive. Essentially, bamboos can be categorized into two types: the invasive or monopodial species, and the non-invasive or sympodial type. The distinction lies in their mode of propagation through rhizomes and stolons, growth patterns, physiology, genetics, and phenotype. This document thoroughly explores the scientific disparities between invasive and non-invasive bamboos.
Keywords: Bamboo; Sympodial; Monopodial; Bambusa; Invasive, Non Invasive.
1. INTRODUCTION
To ascertain whether a bamboo species is invasive or not, a clear understanding of the differences between monopodial bamboos (invasive) and sympodial bamboos (non-invasive), as well as other technical concepts, is essential. Bamboos exhibit remarkable diversity, comprising 1645 species ranging from herbaceous to medium and giant sizes. Giant bamboos, in particular, have gained immense global importance. Deeper exploration of their various facets has revealed their significance not only from a social perspective but also economically. Their potential applications encompass large-scale projects such as biomass production, pellets, bioethanol, engineered wood, industrial charcoal, edible and biodegradable sprouts, cellulose, paper, cogeneration, and more. These ventures, achievable through bamboo, have piqued significant interest worldwide, especially in the Americas. The utilization of giant bamboos in sustainable alternatives has prompted numerous countries to consider them as a viable option for biomass generating farms, intended for commercial use. However, challenges have arisen in the form of permitting their establishment. Often, due to perhaps a lack of understanding regarding bamboo morphology and growth patterns, approvals have been delayed, with the argument being that bamboos are invasive.
Bamboos are categorized as invasive or non-invasive based on factors such as their underground growth patterns, the presence or absence of nodal buds on their stems, and their genetic makeup. Despite sharing similar environmental properties, sympodial bamboos, classified as non-invasive, are preferred for establishing commercial biomass-generating farms. They are favored because of their higher production rates and their inability to spread autonomously and invade other ecosystems. The classification of bamboos into invasive (monopodial) and non-invasive (sympodial) will be described and analyzed in depth during the course of this review. Analyze in depth the differences between an invasive bamboo (monopodial) and a sympodial bamboo (non-invasive) based on its type of rhizomatic growth, flowering, length of rhizomes and stolons, presence of nodal buds, anatomical compositions, places of growth, chemical compositions, space that they occupy on the ground to demonstrate that sympodial bamboos.
Bamboo plant description
In general, a giant bamboo plant including monopodial and sympodial, such as Bambusa vulgaris var vulgaris, is formed by roots, rhizomes or stolons, culm or aerial stem, apical branches, basal branches in some species, cauline and typical leaves, flowers, and fruits if they bloom. The big difference between them is the underground organ called rhizome and stolons, which differ in size, length, shape, and the way they naturally colonize new surfaces. Stolons and rhizomes are essentially the morphological structures that determine whether a bamboo species is monopodial (invasive) or sympodial (not invasive).
Types of bamboo depending on their underground propagation organs and buds
Before starting to define the differences between a sympodial bamboo and a monopodial one, it is strictly necessary to know different botanical concepts that must be applied to sympodial and monopodial species. Botany is a science that studies the structure, organs, characteristics, properties and relationships of plants and their vital processes. This botanical definition allows to clearly identify how the asexual organs used by underground bamboos make them invasive or non-invasive.
The rhizome is a modified stem that grows underground, normally short, thick, compact, strong, which in bamboos turns upward and produces aerial stems. These rhizomes have lateral buds that can produce new rhizomes and aerial stems. Also, in their ventral part they produce thousands of roots that are responsible for extracting nutrients and water. The main functions of the rhizomes are to reserve nutrients and to propagate asexually if necessary. The regeneration of this type of organs through their buds produces stems grouped in a circular shape around each other. These groupings are called clumps. Sympodial bamboos have this kind of organs in the soil. This kind of bamboos are called no invaders. The stolon is a modified stem that grows underground, in the shape of a tube, in bamboo normally with long length, which in bamboos can grow to great lengths, usually hollow, with the presence of buds which activate and generate new stolons and stems. The main functions of the stolons are under its natural regeneration create new stolons and stems and, also create roots so that they extract water and nutrients from the soil and, through their buds that are distant or very distant from each other, producing lonely aerial stems. The regeneration of this type of structures produces new stolons and aerial stems without any arrangement. Stolons are aggressive structures that spread asexually, growing rapidly to colonize surfaces. Monopodial bamboos have this kind of organs in the soil. This kind of bamboos are called invaders. On the other hand, in plants, buds signify the potential of a new plant when it is propagated. In relation to the number of buds that can be activated naturally to form new colonizing plants, there is an enormous difference between sympodial and monopodial bamboos.
Sympodial bamboos have at least 8 buds per rhizome and in addition, each aerial stem that forms the clump has a bud in each node. This enormous number of buds that this type of bamboo has, around 2,280 buds per clump, allows them to be not aggressive in its natural propagation because it has a huge reserve of propagative structures that it only uses in case its normal growth is interrupted. These buds are always in a dormancy state and are only activated if a human propagates it or when the plant is affected in its normal development. This type of bamboo uses very few buds for its development.
Monopodial bamboos have buds in their stolons that they use more to produce new stolons and fewer aerial stems. On the other hand, aerial stems do not have nodal buds, which represent the potential of new plants, which makes them more vulnerable to their elimination; therefore, for the purposes of conservation of the species, they have specialized in colonizing surfaces more quickly (Cruz-Rios, 2005). In all bamboo species, monopodial or sympodial, underground rhizomes or stolons interconnect individual plants. As a result, bamboo forests consist of thousands of plants, with only their aerial organs—stems, branches, and leaves—visible above the ground. In some bamboo species that bloom, the flowers, after pollination, develop into fruits resembling rice grains. Bamboo forests and the aerial placement of their stems depend on the type of the propagation organ they have underground and the ramification occurring into the soil.
Rhizomes and stolons are typically underground and those organs constitutes the structural basis of the plant. Since this part of the plant is completely or almost completely out of sight, this system is one of the least understood components of bamboo. However, not knowing this system can have unfortunate consequences, since the rhizome - stolon system has important functions in the life of the plant. The ramification, along with the resulting placement of aerial stems, categorizes bamboo species into three types: monopodial, sympodial and amphipodial.
Monopodial bamboos
Monopodial bamboos, also known as leptomorphs, which means tube-shaped, feature a long, thin underground rhizome that gives rise to thicker stems. The stolon has single buds, which when activated, sprout into separate stems with the same degree of separation between the buds and stems. The rhizome spread through lateral or monopodial ramifications, forming extensive underground networks that can colonize large areas. This rhizome group contains those species of bamboo which can spread over long distances; thus, they are invasive by nature. (McClure, 1966). Typically, the diameter of a rhizome is smaller than the culm it produces. The internode, or the space between nodes, is longer, relatively uniform in length, typically hollow, and interrupted by diaphragms. Nodes in some genera are elevated or inflated, others do not present lateral buds and feature boat-shaped nodes when dormant (Fangchun, 2001). The rhizomes are tubular in shape, between 1 and 3 cm wide and without determined lengths, sometimes reaching long distances over 6 m. The monopodial bamboo rhizome can be extended horizontally under the soil depending on the species, and its length ranges from 50 to 70 m for Phyllostachis heteroxycla, 90 to 250 m for P. viridis, and 200 to 350 m for P. nigra (Jinghua, 2000). Previous authors mention rhizomes as propagation organs in monopodial bamboos, but as seen previously, botanically these structures are stolons (Cruz-Rios, 2005). This type of bamboo is found in temperate and warm regions but is resistant to freezing temperatures and does well in regions with mild winters. Monopodial bamboos are widely distributed throughout Eastern Asia, particularly China and Japan. The monopodial bamboo is characterized as having strong frost resistance and is distributed in area of higher latitudes, such as Japan, Korea, Yellow River and Yangtze Valley where there is a slight winter (Hwang and Ma, 1994; Zhang et al., 1997; Li et al., 2005). The typical genera of monopodial bamboos are Phyllostachys, Arundinaria, Sasa, Semi-arundinaria, Shibatea and Sinobambusa. Monopodial bamboo species are faster growing than sympodial (Maoyi, 1996). Due to this capability, monopodial bamboos are commonly referred to as running bamboo. The most important monopodial bamboo species are: Phyllostachys pubescens, P. bambusoides, P. praecox, P. nigra, P. aurea, P. machinoi (Hamid et al., 2023).

Figure 3. Example of monopodial bamboo. A) running stolons long and thin. B) monopodial bamboos presenting singles stem
Sympodial bamboos
This type of bamboo, also called pachymorph, such as Bambusa vulgaris var vulgaris, is distinguished in the field because their plants develop in an agglutinated manner, creating strains with growth determined by the development of the plants and the species. In general, this conglomerate of plants, united by their rhizomes, grows in contiguous circles, creating the clump. In sympodial bamboos, the rhizomes are compact, very short or short, thick, and strong, reaching an average length of 22.3 cm and a thickness of 12cm when the forest is mature. The clump in this type of bamboo develops between 4 and 6 years, reaching a maximum of 1.5 m in radius (Cruz-Rios, 2005). The sympodial rhizome has 6 to 7 large lateral buds on either side of the thick rhizome proper, and the buds grow up to new bamboos with a short rhizome neck. The rhizome internodes are broader, long, solid, and asymmetrical, while the nodes are not elevated. The underground rhizome consists typically of two parts: the rhizome proper and the rhizome neck. The neck is basal to the rhizome proper, generally shorter in length and obconical in shape. It connects the new rhizome to the mother rhizome. Rhizomes are usually more or less curveshaped and rarely straight, with maximum thickness, typically somewhat greater than that of the culm (Liese and Kohl, 2015). Sympodial bamboo rhizome, such as Bambusa tulda, can only be extended under the soils, up to 5.2 m in diameter (White and Childers, 1945). Are widely distributed throughout the warmer regions of the tropics in China, Indonesia, Philippines, Thailand, south of India and Latin America and does not tolerate freezing temperatures. The typical genera of sympodial bamboos are Bambusa, Dendrocalamus, Elystrostachys, Gigantochloa, Oxitenanthera and Guadua. Sympodial bamboos take longer to grow than monopodial bamboos but generate greater biomass per unit area (Cruz-Rios, 2009). Due to this capability sympodial bamboos are commonly referred to as clumping bamboos. All the points presented above, emphasizing that the rhizome is small and short and that a strain grows or colonizes very little space, demonstrate how Bambusa vulgaris is not invasive. Furthermore, throughout the document it continues to be demonstrated, especially when talking about flowering and places where it lives, that this species itself is not invasive.
The most important sympodial bamboo species are: Bambusa textilis, B. chungii, B. vulgaris var vulgaris, B. vulgaris var vitatta, B. oldhamii, B. blumeana, B. heterostachys, B. balcooa, B. tulda, B. tuldoides, B. stenostachya, B. spinosa, Dendrocalmus giganteus, D. brandisii, D. latiflorus, D. asper, D. sinicus, Guadua angustifolia, Gigantochloa thoii, G. scortechini, G. ligulate, G. levis, G. ligulate, G. verticilata, G. wrayi, Oxinanthera abyssinica, O. nigrociliata, Schizostachyum brachlycladum, S. funghomii, Tyrsostachys siamensis, T. oliveri and Teinostachyum dulloa
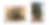
Figure 4. Example of sympodial bamboo. A) clumper rhizomes which are short and compact. B) sympodial bamboo (Dendrocalamus asper) forming clumps.
Amphipodial bamboos
Amphipodial bamboos exhibit a combination of growth patterns, displaying characteristics of both a) monopodial and b) sympodial types. A tube-shaped rhizome initially grows, generating a strain. Subsequently, several monopodial rhizomes sprout, generating sympodial growth. Typically, this type of bamboo is of low to medium height. As discussed above, Bambusa vulgaris has no relationship with this type of growth in bamboos. Amphipodial, which is a combination of monopodial and sympodial rhizomes, belongs to the genera including Bashania and Shibataea (Maoyi, 2007). These genera originated from Japan. The genus most associated with amphipodial bamboo in America is Chusquea, as Chusquea fendleri and C. culeaou. The most important amphypodial bamboo species are: Araundinarea racemosa and Melocanna bacifera.

Figure 7. A) Example of amphypodial bamboo: Chusquea coleaou. B) Vertical and horizontal grow of Amphypodial bamboo
How monopodial and sympodial bamboo grow
From how a bamboo plant grows underground and the shape it has above, it is clearly known whether bamboo is invasive or not. In their development, monopodial bamboos grow in a very different way, naturally colonizing large surfaces presenting themselves with invasive characteristics, while sympodial bamboos in their development colonize very small areas presenting non-invasive characteristics. Moreover, the different types of growth between these two classes of bamboo allow them to be clearly distinguished at the field level (Cruz-Rios, 2009).
Growth in monopodial bamboos
When a small seedling of a monopodial bamboo is planted, it begins to generate tube-shaped stolons with sizes and growth according to its height. These stolons with buds present activate an aerial culm or stem. Subsequently, new slightly larger stolons are generated that cover more space and so on until achieving aerial stems as defined by the species. The stolons rapidly spread underground in unpredictable directions. A single seedling planted of this type, over time, and through the growth capacity of the rhizomes, can naturally colonize large areas. The new shoots or culms can emerge at considerable distances from each other, presenting this type of bamboo as invaders (Cruz-Rios, 2009). In a natural forest or a monopodial bamboo farm, independent stems are observed separated from each other at different distances. On average, the population density of a giant and mature monopodial bamboo is 3000 plants/ha which means that each plant needs around 3.33 square meters of living space. Monopodial bamboos demand a large area for plant development, contributing to their invasive nature. Monopodial bamboos are called runners.
Growth in Sympodial bamboos
Sympodial bamboos create clumps. A clump is a group of interconnected individual plants that are genetically identical, generated by continuous and repetitive propagation of their short and thick rhizomes. It represents a cluster of individuals of the same species grouped into a colony or crop. A giant bamboo clump results from a 10 mm diameter stake and 30 cm tall seedling that starts a propagation process to generate several seedlings 2 mm in diameter, which in turn produces several 5 mm seedlings. Then, the latter generate 1 cm plants that give rise to 2 cm plants, and so forth until plants 10 cm in diameter and 20 m tall are produced. These final diameters and heights are only achieved under adequate climate, soil, and silvicultural management conditions. From its rhizome the seedling initiates the process by creating new seedlings, which in turn produce more plants and all of them form a clump of plants having a great diversity of ages, diameters, and heights. A clump is then the result of a seedling always developing new individuals toward clump periphery in circles in tropical bamboos. The oldest individuals having the smallest diameters and heights are always found toward the clump center, while the youngest ones with bigger diameters and heights are located toward the clump periphery. The following rule governing a clump: under normal development conditions, new plants will always generate new offshoots with bigger rhizomes, thicker diameters, and taller aerial stems from underground rhizomes (Cruz-Rios, 2009). These clumps do not spread further sideways after reaching a certain size, making them non-invasive by nature. Bambusa and Dendrocalamus are essential genera in this category.

Figure 8. Imaginary sympodial bamboo clump development and b) real sympodial clump development. (Cruz-Rios, 2005).
The following figures help to clearly understand the way in which bamboos spread underground, showing how some are clumpers (no invasives) and others are runners (invasives). Despite being in the same tribe, (Bambuseae) the growth and development phases in monopodial and sympodial bamboos, the main difference between them is the asexual propagation organ that they use as a means of colonization below the ground. Sympodials have short and thick rhizomes as a means of asexual propagation and the monopodials have long tube-shaped stolons as way to asexual propagation, also fast growing and with a high capacity for invasion.
In established forests, whether with a protective or commercial nature or that fulfill both functions, monopodial and sympodial bamboos retain their same rhizomatic or stolnes branching pattern, expressing this natural behavior in the final arrangement of the plants in the field. The monopodial bamboos will present individual plants in the established farm that can appear anywhere, dispersed and without any arrangement, while the sympodial bamboos present the plants grouped together in clumps with easily visible arrangements in the field determined by the distances that the farm has been planted.
One hectare planted with monopodial bamboo, due to its stolon pattern of growth, can become several over times, showing the high colonization capacity of this type of bamboo, which is why it is invasive, while a hectare planted with sympodial bamboo, due to its rhizomatic pattern, over time will be the same hectare planted, showing the little capacity for natural colonization that this type of bamboo has. This low capacity, or need to colonize, means that this type of bamboo is not invasive (Cruz-Rios, 2009).
Living space in monopodial bamboos
The classification of culm diameter and height is useful in helping to identify the growth factors for individual species in different site location, topography, climate, and other conditions. (Fangchun, 2001) classified the bamboo growth according to the average diameter namely: Class 1 (diameter more than 12 cm), Class 2 (10 to 12 cm), Class 3 (8 to 10 cm), Class 4 (6 to 8 cm), and Class 5 (less than 6 cm).
The majority of the monopodial bamboo species are classified as class 4 and 5, except for Phyllostachys pubescens (Class 2). This species records the longest culm (21.4 m) for monopodial bamboo. The P. pubescens is cultivated in the Shi Zhua site in Taiwan, where the temperature is lower (11.5°C) and the elevation is higher. In this location, the bamboo exhibits significantly larger measurements, including a higher Diameter at Breast Height (DBH) of 10.6 cm, a greater height of 21.4 meters, and a higher culm density of 8344 culms/ha. In comparison, the Hui sun site, with a temperature and elevation of 20.3°C and 667 meters respectively, shows smaller dimensions: 6.8 cm DBH, 10.3 meters in height, and a culm density of 7933 culms/ha This sindicates that the P. pubescens thrives in mountainous climates, particularly those with elevations ranging from 1000 to 1500 meters. In Phyllostachys edulis forest the population density is between 3,200 and 4,500 stems/ha, which represents a living space per plant between 3.12m2 and 2.2 m2 per plant (Cruz-Rios, 2005). P. edulis is one of the most important bamboos worldwide.
Living space in sympodial bamboos
Clump size influences the culm DBH, and height in sympodial bamboo. Generally, the culm’s DBH and height increases with increasing clump size. The small (4.5 m2 ), medium (7.13 m2 ), and large (9.3 m2 ) clump sizes of B. stenostachya produce the DBH (8.7 cm, 9.3 cm and 10.2 cm) and height (15.3 m, 17.4 m, and 20.3 m) respectively (Chen et al., 2012).
The sympodial bamboos have all the classes in the DBH classification. The species with diameter class 1 including D. asper, D. latiflorus, G. levis, Melocanna bambusoides and Oxytenanthera abyssinica. Others, including B. vulgaris var. striata, B. stenostachya Gigantochloa scortechinii, G. wrayi, and S. grande are classified as class 2, the same as P. pubescens. The D. latiflorus and T. oliveri are recorded as the longest culm (25 m) for sympodial bamboo. This is followed by B. vulgaris var. striata, D. asper, and M. bambusoides (23 m), and lastly, by B. stenostachya, G. ligulata, and S. funghomii (20 m).
A sympodial bamboo plant requires minimal space for growth. A fully developed Bambusa vulgaris clump, that is more than 70 years old, with stems averaging 9.8 cm in diameter and 18.9 m high, has on average between 60 and 80 culms in an agglutinated form that occupy a space of 7.06 m2 , 3 m in diameter, of which each plant needs between 0.12 m2 and 0.09 m2 as living space (Cruz-Rios, 2009). Very few clumps over 70 years old, developing solitary, reach between 4.0 to 5.0 m in diameter.
Other important differences between monopodial and sympodial bamboos
Climates
The sympodial bamboo, on average, (9.3 cm and 17 m) has a significantly higher DBH and height than monopodial bamboo, on average (5.6 cm and 10.8 m). This factor may be due to the fact that most sympodial bamboo are grown in tropical countries that are rich in sunlight and rain for photosynthesis. The starch stored in parenchyma is used for the culm growth. By contrast, most monopodial bamboo is grown in temperate countries with less sunlight, and the starch are stored in the parenchyma that are later used for their sustenance during winter and snow (Hamid et al., 2023).
Bamboo genetics
Despite being in the same tribe, the growth and development phases in monopodial and sympodial bamboo culms may be different due to different rhizome structures. Zhao et al. (2014) made comparison between the micro Ribonuleic acid (mRNA) of monopodial bamboo (Phyllostachys pubescens) and sympodial bamboo (Dendrocalamus latiflorus).
In their reports, the rhizome of monopodial bamboo can spread laterally while grown in soil, and can also be separated from the mother plant, while sympodial bamboo grows in clusters within a relatively small range. The result indicates that there are 19,295,759 and 11,513,888 raw sequence reads, in which 92 and 69 conserve miRNAs, as well as 95 and 62 novel miRNAs are identified in P. pubescens and D. latiflorus, respectively. The ratio of high conserved miRNA families in D. latiflorus is more than that in P. pubescens. In addition, a total of 49 and 106 potential targets are predicted in P. pubescens and D. latiflorus, respectively, in which several targets for novel miRNAs are transcription factors that play important roles in plant development. Experiments show that miR397, miR1432, and miR7748 are specifically conserved in the leaf sample of P. pubescens. Taken together, the comparison between P. pubescens and D. latiflorus indicate that monopodial and sympodial bamboo may share different miRNAs and target genes to have a better adaption for their development in different stages, and stress response in their diverse course of evolution. Therefore, monopodial bamboo requires more self-regulation to adapt to the environment than sympodial bamboo, which might be consistent with the generation of lower conserved miRNAs families. Fangchun (2001) investigated the physical properties of 96 bamboo species and the mechanical properties of 65 bamboo species from both monopodial and sympodial bamboo. The moisture content, density, shrinkage, tensile, and compression properties vary by species, which originated from different rhizome type. The growth, development, and properties of some bamboo species either from monopodial and sympodial rhizomes may also be influenced by the site condition and climate of a specific area.
Sprout and growth
The sprouting and growth phases are significantly shorter in monopodial bamboo (32.7 and 33.2 days) compared to the sympodial bamboo (105.8 and 102.6 days), respectively. The bamboo shoot elongation rate within 24 h depends on the genera, species and rhizome. Phyllostachys reticulate records the fastest growth rate (maximum 120 cm/day) for monopodial bamboo (Ueda, 1960), and this rate is the same as that of Dendrocalamus asper for sympodial bamboo (Subsansenee, 1994). The growth rate of Bambusa balcooa, Dendrocalamus gigantus, and Bambusa vulgaris are recorded as 77 cm/day, 58 cm/day, and 44 cm/day, respectively (Osmastos, 1918; Banik, 1993).
Anatomical vascular bundle
The radial length and tangential diameter of the vascular bundle is significantly higher in monopodial bamboo (0.39 μm and 0.37 μm) than sympodial bamboo (0.28 μm and 0.22 μm), respectively, while the R/T ratio of vascular bundle is significantly higher in sympodial bamboo (1.23) than the monopodial bamboo (1.07), the metaxylem vessel diameter is significantly higher in sympodial (127.97 μm) than the monopodial bamboo (72.38 μm). In contrast, the vascular bundle frequency is significantly higher in monopodial (5.18/mm2) than the sympodial bamboo (2.80/mm2). The proportion of metaxylem vessel diameter is significantly higher in sympodial (6.40%) than the monopodial bamboo (5.21%), but not for fiber and parenchyma proportions. The proportion of fiber and parenchyma are not significantly different for monopodial (40.92% and 45.56%) and sympodial (53.00% and 53.38%) bamboos, respectively (Jawaid et al., 2023).
In monopodial bamboo, I. migoi and P. pubescens growing in China have the longest fiber (2250 μm), while the Indocalamus tessellatus grown in China has the shortest fiber (1435 μm). The Brachycladum densiflorus grown in China records the widest fiber (16.9 μm), while the Arundinaria amabilis also grown in China has the thickest fiber wall, and the P. viridis grown in USA has the widest fiber lumen (5.63 μm). The fiber of the three monopodial bamboos grown in Taiwan is the longest, when ranked in descending order of Phyllostachys bambusoides (2033 to 2239 μm), P. nigra (1934 to 2199 μm), and P. pubescens (1375 to 1573 μm). The outer zone has a longer fiber than the inner zone for all species (Jeon et al., 2018).
In sympodial bamboo, the longest and shortest fibers are recorded for G. scortecinii (4240 μm) grown in Malaysia and B. basihirsuta (1667 μm) grown in China, respectively. The fiber is widest in B. heterostachya (26.8 μm) grown in Malaysia and narrowest in D. striticus (9.9 μm) grown in China. The fiber wall is thickest in G. thoii (12.2 μm) grown in Malaysia and thinnest in D. giganteus (1 μm) grown in Thailand. The fiber lumen is largest in B. blumeana (10 m) grown in Malaysia and smallest in Sinarundina chungi (1.59 μm) grown in China. The fiber wall diameter, thickness, and lumen diameter of B. blumena, B. vulgaris, and G. scortechinii are not significantly different with culm aged one to three years. The fiber length ranges from 1.89 μm to 1.99 μm in B. blumeana, from 3.30 μm to 3.76 μm in B. vulgaris, and from 3.50 μm to 4.24 μm in G. scortechinii. The fiber diameter ranges from 0.018 μm to 0.02 μm in B. blumeana, 0.017 μm in B. vulgaris and G. scortechinii. The fiber wall thickness is 0.05 in B. blumeana, ranges from 0.06 to 0.07 in B. vulgaris, and 0.07 to 0.08 in G. scortechinii. The fiber lumen diameter ranges from 0.009 to 0.010 in B. blumana, from 0.002 to 0.003 in B. vulgaris, and G. scortechinii (Mohmod et al., 1990).
Analyzing the fiber morphology data shows that the fiber length and diameter are significantly longer and wider in sympodial bamboo (2494.8 μm and 17.06 μm) than the monopodial bamboo (1929.7 μm and 12.95 μm), respectively. In contrast, fiber lumen diameter and wall thickness are not significantly different in monopodial (3.63 μm and 3.93 μm) and sympodial (4.49 μm and 4.39 μm) bamboos, respectively (Alomar et al., 2023).
2. CONCLUSIONS
Sexual seeds are the main organs of invasion or colonization of plant species, but in the case of sympodial bamboos, although it can sometimes flower, there is no seed production, which makes this species incapable of colonizing or invading new areas using sexual seeds.
Monopodial bamboos are invasive because they have as an asexual propagation organ, a stem modified into stolons, which are tube-shaped structures, long or very long, with rapid growth and a high capacity to invade large areas in a short time.
Sympodial bamboos such as Bambusa vulgaris are not invasive due to the asexual propagation they have underground is the modified stem called rhizome that are short-growing, compact, grouped structures with the capacity for self-controlled invasion. In relation to the number of buds that can be activated naturally to form new colonizing plants, there is an enormous difference between sympodial and monopodial bamboos. Sympodial bamboos, have at least 8 buds per rhizome and in addition, each aerial stem that forms the clump has a bud in each node. This enormous number of buds that this type of bamboo has, around 2,280 buds per clump, allows them to be not aggressive in its natural propagation because it has a huge reserve of propagative structures that it only uses in case its normal growth is interrupted.
Monopodial bamboos have buds in their stolons that they use more to produce new stolons and fewer aerial stems. On the other hand, aerial stems do not have nodal buds, which represent the potential of new plants, which makes them more vulnerable to their elimination; therefore, for the purposes of conservation of the species, they have specialized in colonizing surfaces more quickly.
For example a fully developed Bambusa vulgaris clump with 10 years old, has 3.6 m of diameter in the soil, which means that it occupies an area of 10.17 m2. Now, if it is considered that a clump of this age has practically stopped its expansion in the soil and that it has an average of 60 plants finely grouped, the conclusion is that the vital space needed by a plant of this species only needs 0.16 m2. This proves that Bambusa vulgaris plant requires minimal space for growth and does not need much space to live. If the population density of a giant monopodial bamboo is 3,000 plants per ha, this means that this type of plant needs an average of 3.33 m2 to live, which is too large a space compared to a bamboo plant that only needs 0.16 m2.
There is an enormous botanical difference between invasive and non-invasive bamboos, which was explained in depth during the article, which allows us to deduce that sympodial bamboos are not invasive. Now, if compared the vital space that Bambusa vulgaris colonizes with respect to another plant species that has nothing to do with bamboos and that is considered non-invasive, a Bambusa vulgaris clump needs 10.17 m2 and every plant of its clump needs only 0.16 m2 , while a sequoia tree, Sequoia sempervirens has an average length of 80 m an occupy a diameter of 4.5 m, which is equivalent to saying that it needs a living space of 15.9 m2 .
REFERENCES
Banik, R. L. (1993). Periodicity of culm emergence in different bamboo species of Bangaladesh. Annals of Forestry 1 (1): 13–17.
Banik, R. L. (2015). Morphology and growth, in: Bamboo. The plant and its uses, W, Liese & M, Kohl. (Eds.), 43-90.
Bharma, S., and Brahma, B. K. (2018). Chemical composition: The prerequisite criteria for bamboo utility, (Study of Bambusa garauchokua, Bambusa pallida and Bambusa assamica of Kokkrajhar district of Btc, Assam, India), Journal for Advance Research in Applied Sciences 5(4), 1-9.
Chen, T. H., Wang, D. H., and Chung, H. Y. (2012). The growth and biomass of Bambusa stenostachya plantation at Zeochen Area in Tainan City, Quarterly Journal of Chinese Forestry 45(3), 341-350. DOI: 10.30064/QJCF.201209.0005
Cruz-Rios Hormilson. (1992). Aproximaciones para las fertilizaciones en Guadua angustifolia. Armenia: C.R.Q.
Cruz-Rios Hormilson. (1994). La Guadua: Nuestro Bambú. Corporación Autónoma Regional del Quindío. Centro Nacional para el Estudio del Bambú-Guadua. Armenia-Quindío.
Cruz-Rios Hormilson. (2002). El Bambú, Bambusa vulgaris, su silvicultura y su uso en la Industria del Alcohol, Almidón, Celulosa y Papel. Editorial COLMEX. Villahermosa, Tabasco, México.
Cruz-Rios Hormilson. (2005). Natural forest and commercial bamboo plantations.
De Vos, V. (2010). Bamboo for Exterior Joinery: A Research in Material and Market Perspectives, B.Sc. Thesis, University of Applied Sciences, Van Hall Larenstein, Leeuwarden, Netherlands.
Fangchun, Z. (2001a). Annual highlights. International bamboo and rattan organization, in: Selected Works of Bamboo Research, The Bamboo Research Editorial Committee, Nanjing Forestry University Publisher, Nanjing, China.
Fangchun, Z. (2001b). Cultivation and Integrated Utilization on Bamboo in China, China National Bamboo Research Centre Publisher, Hangzhou, China.
Fei, B., Gao, Z., Wang, J., and Liu, Z. (2016). Biological, anatomical, and chemical characteristics of bamboo secondary xylem biology, Secondary Xylem Biology, Y. S. Kim, R. Funada, and A. P. Singh (eds.), Academic Press, Cambridge, MA, USA, pp. 283-306. DOI: 10.1016/B978-0-12-802185-9.00014-0
Grosser, D., and Liese, W. (1971). On the anatomy of Asian bamboos, with special reference to their vascular bundles, Wood Science and Technology 5, 290-312. DOI: 10.1007/BF00365061
Grosser, D., and Liese, W. (1973). Present status and problems of bamboo classification, Journal of the Arnold Arboretum 54(2), 293-308. DOI: 10.5962/p.184524
Hagarth, N. J., and Belcher, B. (2013). The contribution of bamboo to household income and rural livelihoods in a poor and mountainous county in Guangxi, China, International Forestry Review, 15(1), 71–81. DOI:10.1505/146554813805927237
Hamid, N. H., Jawaid, M., Abdullah, U. H., and Alomar, T. S. (2023). Monopodial and sympodial bamboos grown in tropic and sub-tropic countries – A Review, BioResources 18(3), 6499-6560.
Jeon, W. S., Kim, Y. I., Lee, J. A., and Kim, A. R. (2018). Anatomical characterization of three Korean bamboo species, Journal of the Korean Wood Science and Technology 46(1), 29-37. DOI: 10.5658/WOOD.2018.46.1.29
Jin, A., Shao, X., Qiu, S., and Zhang, F. (1999). Biological characteristics of Phyllostachys heteroclada, Journal of Zhejiang Forestry College 16(3), 238-241.
Ju, Z., Zhan, I., Cui, J., Brosse, N., Zhang, H., and Hong, L. (2021). Eco-friendly method to improve the durability of different bamboo (Phyllostachys pubescens, Moso) sections by silver electrochemical treatment, Industrial Crops and Products 172, DOI: 10.1016/j.indcrop.2021.113994
Li, W., Liu, M., Zhai, H., Wang, H., and Yu, Y. (2020). Preparing highly durable bamboo materials via bulk furfurylation, Construction and Building Materials 262, DOI: 10.1016/j.conbuildmat.2020.120726
Li, X. (2004). Physical, Chemical, and Mechanical Properties of Bamboo and its Utilization Potential for Fiberboard Manufacturing, Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA.
Li, Z. (2003). Diversity and Ecology of Mountain Bamboos in the Shennongjia National Reserve of Central China Implications for Resource Management and Biodiversity Conservation, Cuvillier Verlag, Göttingen, Germany.
Li, Z. C., Fu, M. Y., and Xu, D.Y. (2003). Bamboo ecosystem and carbon dioxide sequestration, Journal of Bamboo Research 4, 1-6.
Li, Z. H., Denich, M., and Porsch, T. (2005). Growth behaviour of Phyllostachys nigra var henonis (Bambusoides) in central China, Journal of Forestry Research 16(3), 163-168. DOI: 10.1007/BF02856808
Liese, W. (1985). Bamboos-Biology, Silvics, Properties, Utilization, TZ- Verlag & Print GmbH, Roßdorf, Germany.
Liese, W., and Weiner, G. (1996). Ageing of bamboo culms. A review, Wood Science and Technology 30, 77-89. DOI: 10.1007/bf00224958
Liese, W. and Kohl, M. (2015). Bamboo. The Plant and Its Uses. Springer, Switzerland. 346p. Maoyi, F., Xiasheng, Y., and Shenxui, J. (2007). Technical Manual on Utilization of Sympodial Bamboos, China Forestry Publishing House, Beijing, China.
McClure, F. A. (1966). The Bamboos: A Fresh Perspective, Harvard University Press, Cambridge MA, USA. Massachusetts. 347 pp
Mariano Sánchez Trocino. (2009). Manual de actualización del manejo nutricional y producción de bambú para el panda gigante (Ailuropoda melanoleuca) y panda rojo (Ailurus fulgens) en el zoológico de Chapultepec, Ciudad de México. Programa de Becas del Centro de Estudios ChinaMéxico, UNAM y el ICyTGDF. 71 p
Mera, F. A. T., and Xu, C. (2014). Plantation management and bamboo resource economics in China, Maderas: Ciencia y Tecnología 7(1), 1-12. DOI: 10.18779/cyt.v7i1.181
Mohamed, J., Hazandy, A. H., Ahmad, A. N., and Nik Mohd, M. N. A. (2019). Chemical attributes of Gigatochloa scortechinii bamboo rhizome in relation with hydraulic conductance, BioResources 14(4), 8155-8173. DOI: 10.15376/biores.14.4.8155-8173
Mohmod, A. L., Tarmeze, W. A. and Fauzidah, A. (1990). Anatomical features and mechanical properties of three Malaysian bamboos, Journal of Tropical Forest Science 2(3), 227-234.
Mohmod, A. L., Khoo, K. C. and Nor Azah, M. A. (1992). Carbohydrates in some natural stand bamboos, Journal of Tropical Forest Science 4(4), 310-316. Mohmod, A. L., Ashaari, A., Jamaluddin, K. and Zim, J. M. (1993). Effect of anatomical characteristics on physical and mechanical properties of B. blumeana, Journal of Tropical Forest Science 6, 159-170.
Mohmod, A. L., Khoo, K. C., Jamaludin, K., and Abdul Jalil, H. A. (1994). Fiber morphology and chemical properties of Gigantochloa scortechinii, Journal of Tropical Forest Science 6, 397- 407.
Mohmod, A. L. and Phang, M. T. (2001). The mechanical properties of Bambusa vulgaris and Gigantochloa scortechinii grown in Peninsular Malaysia, Journal of Tropical Forest Products 7(1), 111-125.
Nordahlia, A. S., Uyup, M. K. A., Husain, H., Mohmod, A. L., and Awalludin, M. F. (2019). Anatomical, physical, and mechanical properties of thirteen Malaysian bamboo species, BioResources 14(2), 3925-3943. DOI: 10.15376/biores.14.2.3925-3943
Norul Hisham, H., Mohmod, A.L. and Sulaiman, O. (2003). Decay resistance of bamboo (Gigantochloa scortechinii) compared to 24 Malaysian hardwood, XII World Forestry Congress, Quebec, Canada.
Norul Hisham, H., Othman, S., Rokiah, H., Abdul Latif, M., Ani, S., and Mohd Tamizi, M. (2006). Characterisation of bamboo Gigantochloa scortechinii at different age, Journal of Tropical Forest Science 18(4), 236-242.
Norul Hisham, H., Othman, S., Azmy, M., and Norasikin, A. L. (2012). The decay resistance and hyphae penetration of bamboo Gigantochloa scortechinii decayed by white and brown rot fungi, International Journal of Forestry Research, DOI: 10.1155/2012/572903
Osmastos, B. B. (1918). Rate of growth of bamboo, India Forester 44(2), 52-57. Raka, I. D. N., Alit Wiswasta, I. G. N., and Made Budiasa, I. (2011). Pelestarian Tanaman
Bambu sebagai UpayaRehabilitasi Lahan dan Konservasi Tanah di Daerah Sekitar Mata Air pada Lahan Marginal di Bali Timur [Conservation of bamboo plants as an effort for land rehabilitation and soil conservation in the area around springs on marginal land in East Bali], Agrimeta 1(1), 1- 11. Ram, N., Singh, L., and Kumar, P. (2010). Bamboo plantation diversity and its economic role in North Bihar, India, Nature and Science 8(11), 111-115.
Razak, W., Mohd Tamizi, M., Othman, S., Aminuddin, M., Affendy, H., and Khalid, I. (2010). Anatomical and physical properties of cultivated two and four years old Bambusa vulgaris, Sains Malaysiana 39(4), 571-579.
Razak, W., Mohd Tarmeze, M., Mohammed Abdus, S., Mahmud, S., Hashim, W. S., and Mohd Sukhairi, M. R. (2013). Chemical composition of four cultivated tropical bamboo in Genus Gigantochloa, Journal of Agricultural Science 5(8), 66-75. DOI: 10.5539/jas.v5n8p66
Tomak, E. D., Topologlu, E., Ay, N., and Yilidz, U. C. (2012). Effect of accelerated aging on some physical and mechanical properties of bamboo, Wood Science and Technology 46, 905- 918. DOI: 10.1007/s00226-011-0454-7
Tomak, E. D., Topaloglu, E., Gumuskaya, E., Yildiz, U. C. and Ay, N. (2013). An FT-IR study of the changes in chemical composition of bamboo degraded by brown rot fungi, International Biodeterioration & Biodegradation 85,131-138. DOI:10.1016/j.ibiod.2013.05029
Wang, J. X., Ma, Z. G., Liu, C. X., and Gan, L. M. (1991). Preliminary study on the growth and development roles of Fargesia denuduta bamboo, Journal of Bamboo Research 10(3), 38-48.
Wang, J., Chen, T. H., Chung, H., Li, T., and Liu, C. P. (2009). Structures, aboveground biomass, carbon storage of Phyllostachys pubescens stands in Huisun experimental forest station and Shi-Zhuo, Quarterly Journal of Forestry Research 31(4), 17-26. DOI: 10.29898/SHBQ.200912.0002
Wang, Y., Zhan, H., Ding, Y., Wang, S., and Lin, S. (2016). Variability of anatomical and chemical properties with age and height in Dendrocalamus brandisiii, BioResources 11(1), 1202-1213. DOI: 10.15376/biores.11.1.1202-1213
White, D. G., and Childers, N. F. (1945) Bamboo for controlling soil erosion, Agronomy Journal 37(10), 839-847. DOI: 10.2134/agronj1945.00021962003700100007x
Zhao, H., Wang, L., Dong, L., Sun, H., and Gao, Z. (2014). Discovery and comparative profiling of microRNAs in representative monopodial bamboo (Phyllostachys edulis) and sympodial bamboo (Dendrocalamus latiflorus), PLoS ONE 9(7), 1-3. DOI: 10.1371/journal.pone.0102375
Zhijun Lu, Scott B. Franklin. (2022). Clonal integration and regeneration in bamboo Bashania fargesii, Forest Ecology and Management, 523, ISSN 0378-1127, DOI:10.1016/j.foreco.2022.120504.
--------------
This scientific paper by Hormilson Cruz published in the "International Journal of Agriculture, Environment and Bioresearch", aligns perfectly with our ongoing project at bamboo bioproducts involving the cultivation of Bambusa vulgaris var vulgaris, a sympodial bamboo often mistakenly considered invasive. As highlighted in this research, sympodial bamboos like Bambusa vulgaris var vulgaris can be utilised as a greener alternative to traditional materials. Learn more about our project here.
